Corrosion of aluminium and stainless steel hot tub stoves
Manufacturers usually produce wood-burning stoves for hot tubs made of aluminium alloys or stainless steel. This tendency can be explained by the fact that these materials have good anti-corrosion properties and are resistant to high temperatures. Let’s consider the anti-corrosion properties of these metals in fresh and saltwater.
There are various types of corrosion and each of them affects the destruction of metal to varying degrees. Therefore, we are going to consider only those that exert the most pernicious effect on metal.
1. Corrosion resistance of aluminium water heating stoves
Aluminium does not corrode in clean water. Unfortunately, tap water contains many impurities and chemical elements that can destroy aluminium. Powerful corrosive elements: fluorine, potassium, sodium. Aluminium and its alloys corrode when exposed to chemical compounds of bromine and chlorine, lime and cement solutions.
The most damaging type of aluminium corrosion is pitting corrosion (see pic). Pitting corrosion is a localized form of corrosion by which cavities or pits of irregular shape are produced in the material. Diameter and depth of the pits depends on chemical composition of aluminium alloy, type of corrosive environment and operating conditions. Pitting corrosion occurs when the metal is in constant or frequent contact with an aquatic environment: fresh water, seawater, rainwater, and humid air.
It has long been known that pitting corrosion of aluminium develops in the presence of chlorides. This means that the risk of pitting is much higher in salt water than in fresh water.
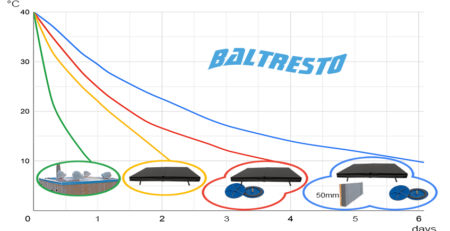
Most of the appearing pits stop their growth within several days. Corrosion products accumulate above the corrosion pit and gradually block the entrance to it. In most cases, the depth of the corrosion pits does not exceed 0,4 mm.

2. Corrosion resistance of stainless steel water heating stoves
Stainless steel water heating stoves have excellent durability and anti-corrosion properties due to their nickel and chromium content. The chromium content in steel contributes to the development of an oxide layer on the surface of the material, protecting the steel from external chemical influences. However, corrosion can occur in the area of the weld due to the burnout of chromium during the welding process.
Stainless steel is resistant to both fresh water from various sources and salt water. However, the abovementioned pitting corrosion of aluminum can also appear on steel under the influence of various external factors and operating conditions.
Rust can also appear in small spots on the metal itself. This happens due to the contact of other metal particles that are not very resistant to corrosion with the metal surface, for example, during the contact with other metallic materials or cleaning with a wire brush. Microparticles get stuck in softer stainless steel and appear as rust spots when interacting with moisture (also contained in the air). Both types of corrosion can be easily removed with a polishing sponge and will no longer show up.